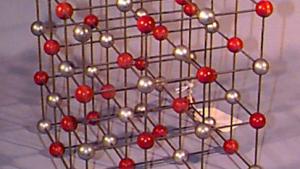
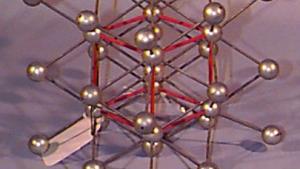
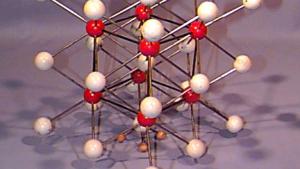
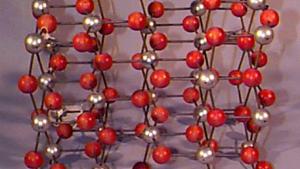
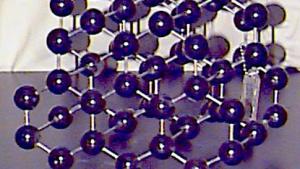
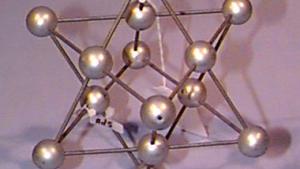
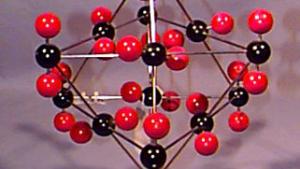
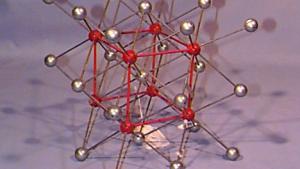
Using physical models to illustrate crystal structures, this demonstration provides a tangible representation of various lattice arrangements. Models of common crystals, such as sodium chloride (NaCl), calcium carbonate (CaCO₃), graphite, and diamond, are displayed to highlight their unique packing and bonding structures. Each model exemplifies different types of crystal lattices, including simple cubic, face-centered cubic, and hexagonal arrangements. This demonstration can also delve into the study of packing factors and their significance in determining the density and stability of crystals. Additionally, a ripple tank with a periodically perforated plate can be used to create periodic wave patterns, mimicking the atomic arrangement found in materials like graphene, further emphasizing the importance of periodicity in crystal structures.