Description
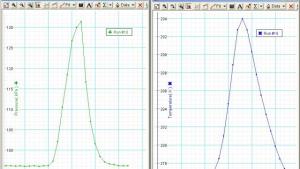
The Ideal Gas Law Syringe demonstration uses a syringe to illustrate the relationship between pressure, volume, and temperature in an ideal gas. By varying the volume of the gas inside the syringe and measuring the resulting changes in pressure, students can observe how the gas behaves according to the ideal gas law, PV=nRT. When the gas is compressed or expanded by moving the syringe plunger, the pressure changes inversely with volume, demonstrating Boyle's Law. Additionally, heating or cooling the gas inside the syringe while keeping the volume constant shows the direct relationship between temperature and pressure, illustrating Gay-Lussac's Law.
PIRA DCS Number
4E00.00
Preparation Time
10 minutes
Preparation & Instructions
There is a white stop built into the syringe. It stops at 20 mL. A small amount of air in the tubing causes an error. Use the formula (V1+V0)/(V2+V0) =P2/P1 where V0 is the air in the tube. There is a response time of about half a second between the pressure and the temperature measurement.